Empirical Formula of Vitamin C
Threonine is an amino acid with the formula C4H9NO3C4H9NO3. If we multiply all the subscripts in the empirical formula by 2 then our molecular formula will be.
Ascorbic acid or vitamin C is a water-soluble organic compound with the.
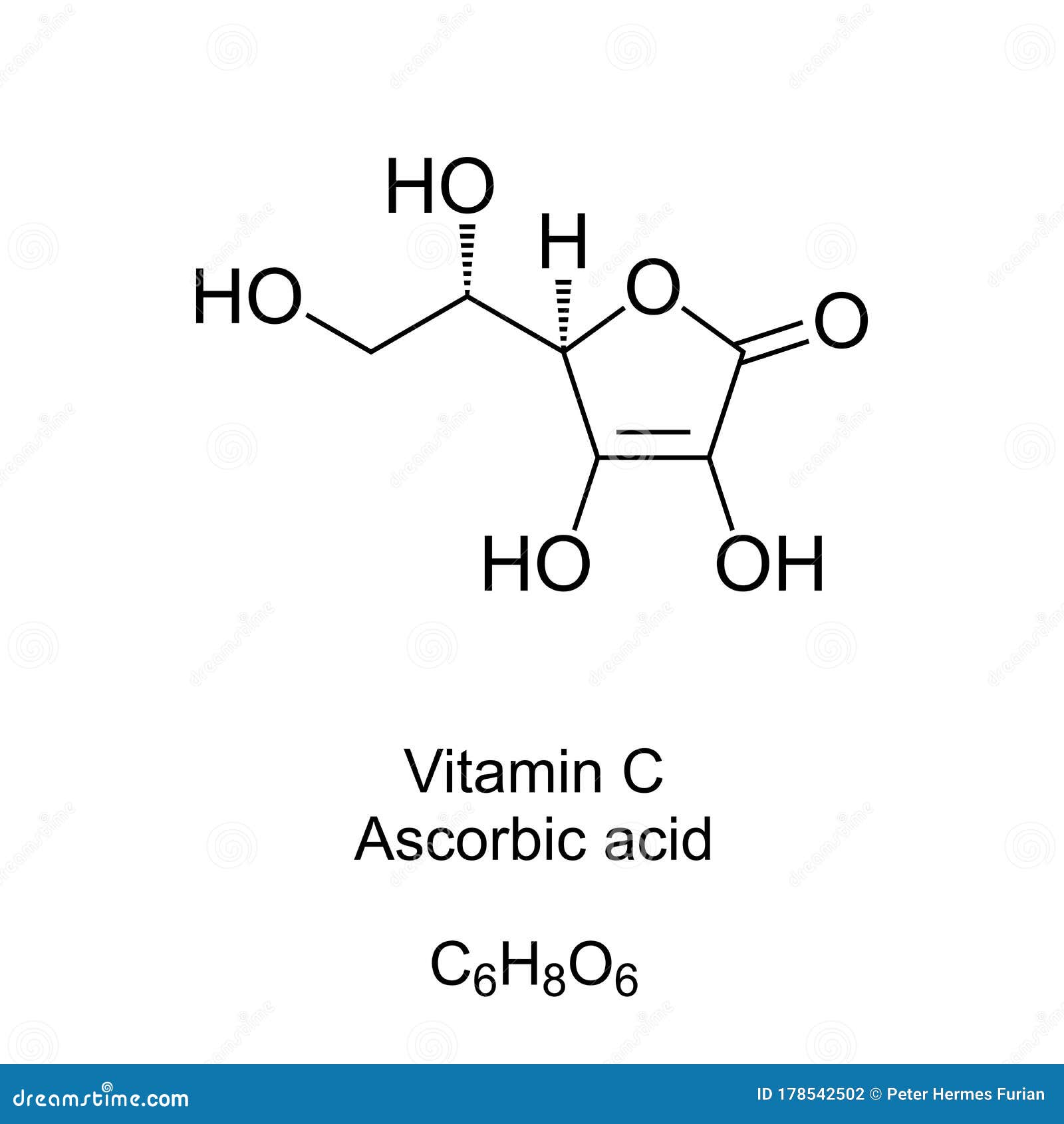
. From this formula we can say that our organic compound is vitamin. The empirical formula of vitamin C is C3H4O3. Determine the empirical formula of vitamin C.
So from that we calculate the molecular mass by performing the calculation multiplying with individual atomic weights - 3x12 4x1. The compound contains six atoms of oxygen. Determine the number of moles of carbon in 4815 g4815 g of threonine.
Of vitamin C is 100 mg and amounts as large as 2 to 3 g are taken by many people without adverse effects. The empirical formula of a chemical compound is the simplest whole-number ratio of atoms present in a molecule. If we multiplied our empirical formula by 2 then the molecular.
Calculate the empirical formula and the molecular formula of vitamin C. A compound of lead has the following percentage composition. What is the empirical formula for vitamin C.
Calculate the empirical formula of the compoundPb 207O16. The composition of ascorbic acid vitamin C is 4092 carbon 458 hydrogen and 5450 hydrogen. You have calculated the following.
When given composition and asked for the empirical formula it is easiest to just assume 100 g of material. The surface of the metal emits a. For example the molecular formula of glucose is C_6H_12O_6 and we do not simplify it into.
What is the molecular formula for vitamin c. Thus Mass C 4092 g. Remember the empirical formula is just the smallest ratio of the elements in the compound.
Moles C 4092 g x 1 mole12 g 341. Thus it would appear that our empirical formula is essentially one half the mass of the actual molecular mass. An empirical formula takes into account only the chemical composition and not the structureExample.
From this information the empirical formula can be calculated. N Number of oxygen in molecular formulaNumber of oxygen in molecular. Its empirical formula is C.
Medium Solution Verified by Toppr Vitamin C contains 4092 carbon by mass 458 hydrogen and 5450 oxygen. 100 g of vitamin C will. 11The Nicotine molecule has been.
Click hereto get an answer to your question What is the empirical formula of vitamin C. An atom of a rare earth element is illuminated at a wavelength of 4882 Angstroms. It is insoluble in water soluble in chloroform.
Pb 9066 O 934. Empirical formula of ascorbic acid. Hence emperical formula of compound is C 2 H 3 O 3.
In a molecular formula it states the total number of atoms of each element in a molecule.
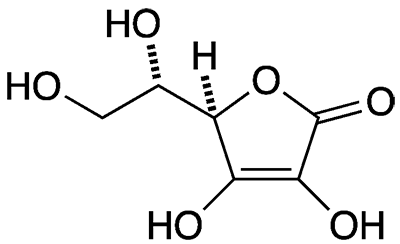
Ascorbic Acid American Chemical Society
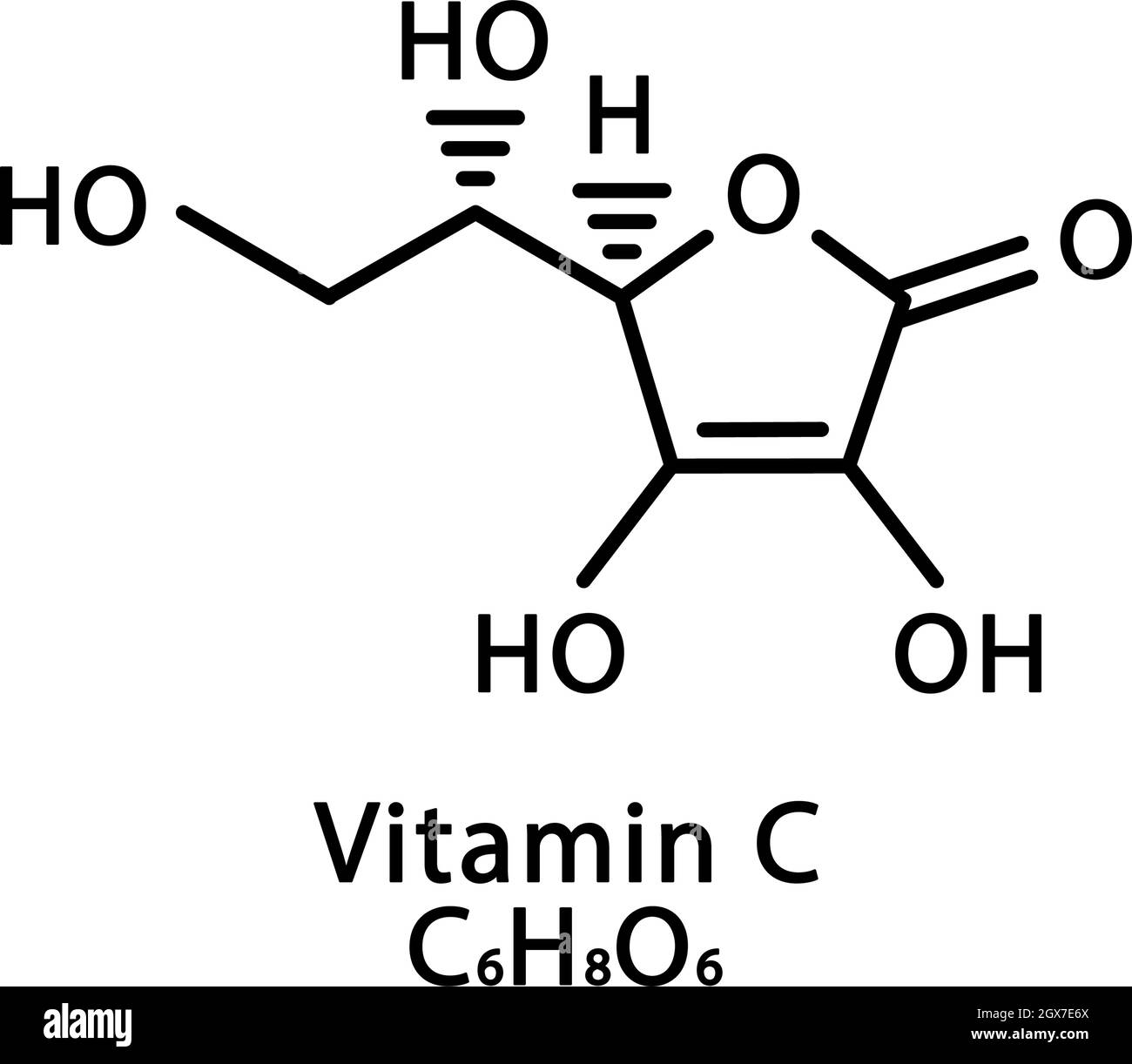
Vitamin C Ascorbic Acid Molecular Structure Vitamin C Ascorbic Acid Skeletal Chemical Formula Chemical Molecular Formulas Stock Vector Image Art Alamy

Vitamin C Ascorbic Acid Skeletal Formula And Molecular Structure Stock Vector Illustration Of Organic Chemical 178542502
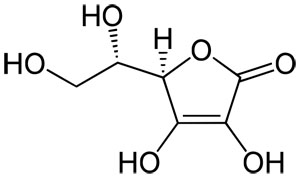
The Vitamin C Molecule Antioxidant Properties
0 Response to "Empirical Formula of Vitamin C"
Post a Comment